Interpreting Genexpert Results: Understanding the Role of MTB and RIF in TB Diagnosis
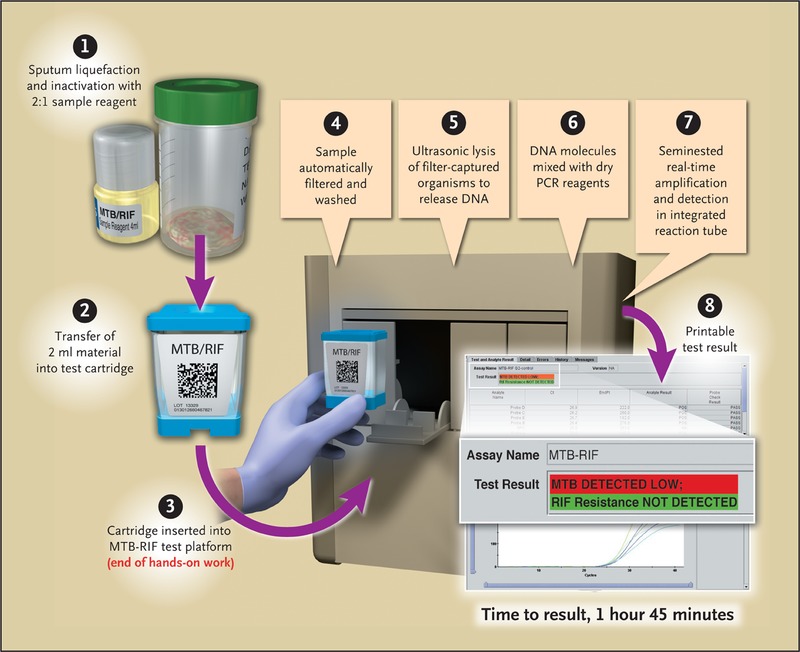
Here is a sample Genexpert MTB/RIF report format:
Patient Information:
Name: [Patient’s name]
Age: [Patient’s age]
Gender: [Patient’s gender]
Medical Record Number: [Patient’s medical record number]
Specimen Type: [Type of specimen collected]
Test Results:
MTB (Mycobacterium tuberculosis) Detected: Yes/No
RIF (Rifampicin) Resistance Detected: Yes/No
Cycle Threshold (Ct) Value: [Numeric value]
Interpretation:
The Genexpert MTB/RIF test was performed on the patient’s specimen and the results are as follows:
If MTB Detected: The presence of MTB DNA was detected in the patient’s specimen, indicating that the patient may have active tuberculosis.
If RIF Resistance Detected: The presence of mutations in the rpoB gene associated with rifampicin resistance was detected in the patient’s MTB DNA, indicating that the patient’s TB strain may be resistant to rifampicin.
If Cycle Threshold (Ct) Value: The Ct value is a measure of the amount of MTB DNA in the patient’s specimen. Lower Ct values indicate higher amounts of MTB DNA and may indicate a more severe or active infection.
Clinical Interpretation and Recommendations:
These test results should be interpreted in conjunction with the patient’s clinical presentation, medical history, and other diagnostic tests. The presence of MTB DNA and/or rifampicin resistance in the patient’s specimen may indicate active TB and/or drug-resistant TB, respectively. The healthcare provider should consider initiating appropriate treatment based on these results and clinical judgment.
It is important to note that this is a sample report format and actual Genexpert MTB/RIF reports may vary based on the laboratory and healthcare provider’s preferences.
| Genexpert MTB/RIF Test Results | MTB Detected | RIF Resistance Detected | Ct Value | Interpretation |
|---|---|---|---|---|
| Active TB with rifampicin resistance | Yes | Yes | Any | The patient has active TB with rifampicin resistance. Appropriate treatment should be initiated based on these results and clinical judgment. |
| Active TB | Yes | No | Any | The patient has active TB. Appropriate treatment should be initiated based on these results and clinical judgment. |
| TB not detected | No | N/A | N/A | TB was not detected in the patient’s specimen. Further testing or evaluation may be required depending on the patient’s clinical presentation and medical history. |
| Technical issue with the test | Error | N/A | N/A | There was a technical issue with the test. The test should be repeated or an alternative diagnostic method should be considered. |
| Active TB with possible rifampicin resistance | Yes | Yes* | Any | The patient has active TB with possible rifampicin resistance. Further testing or evaluation may be required to confirm the resistance. |
| No active TB detected, but patient may have latent TB or history of TB treatment with rifampicin | No | Yes | Any | The patient does not have active TB detected, but may have latent TB or a history of TB treatment with rifampicin. Further evaluation may be required. |
*Note: If RIF resistance is detected with a low confidence level or with mutations outside of the usual rpoB gene region, further testing may be required to confirm the resistance. Ct value may vary depending on the amount of MTB DNA in the patient’s specimen.
Leave a Reply