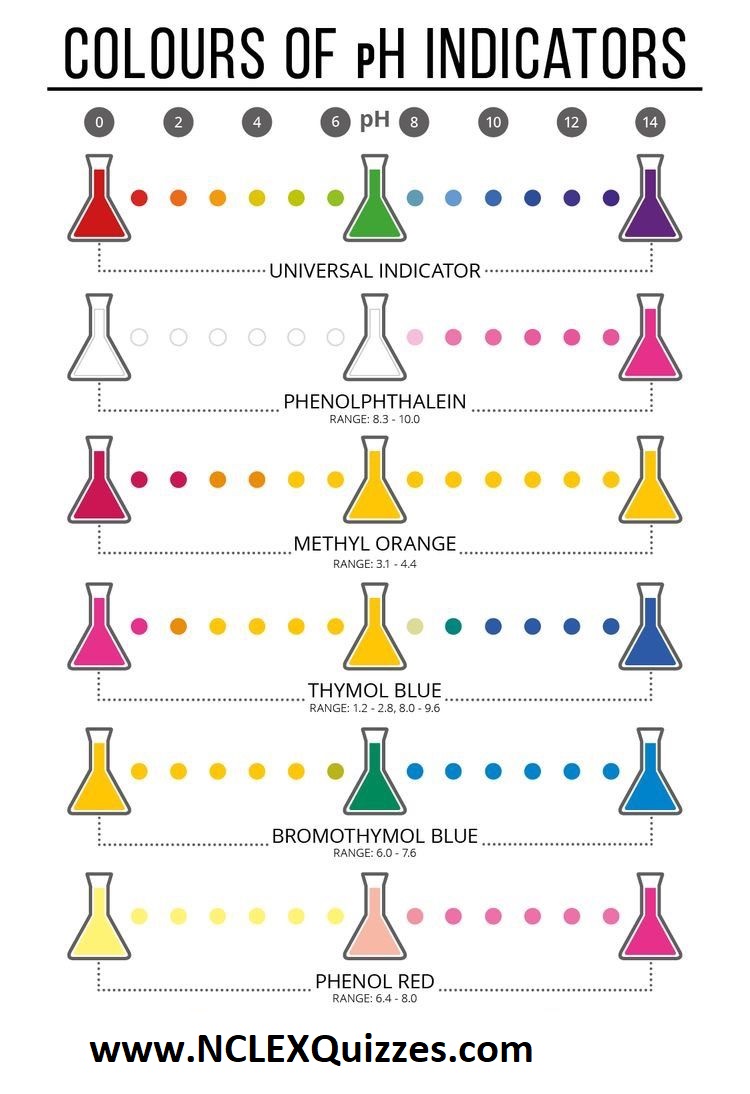
Acid Base indicators (also known as colours of pH indicators)
The pH scale runs from 0 to 14, with each number assigned a different color. At the bottom of the scale sits red, which represents the most acidic, and a dark blue at its opposite end represents 14 and alkalinity. In the middle zone, the pH scale becomes neutral. Milk has a pH of 6 and a neutral off-white color.
pH indicators detect the presence of H+ and OH-. They do this by reacting with H+ and OH-: they are themselves weak acids and bases. If an indicator is a weak acid and is coloured and its conjugate base has a different colour, deprotonation causes a colour change.
Leave a Reply